Question #539df
2 Answers
The size of the group decides whether a molecule is bulky or not.
 )
)
Consider the methane molecule. It has 4 C-H bonds arranged in the 3D space and having a bond angle of 109.5 degrees.Note that these bonds are free to rotate in space as long as they remain in tetrahedral geometry.
Now i replace the three Hydrogen atoms with Methyl groups.Try to imagine the structure of the molecule- the same carbon at the centre attached to 4 carbons which in turn are attached to the 3 hydrogen atoms each.
Look at methane and see how the H are arranged in the entire 360-degree space.Now for the new molecule try to imagine how much space is available for the 12 hydrogen atoms (3 from 4 carbons) to rotate.Hardly any.In fact they will try to repel each other off.This is how we define a bulky group.Bulky groups do not allow the entry of another atom from another molecule easily(since they leave you no space to approach the central atom).
Quick answer: The t-butyl group is a bulky group.
"Bulky" is a relative term. The real question is, "Bulky compared to what?"
The bulk of a substituent is often measured by its effect on the rate of an
This gives an order of bulkiness:

The typical bulky base is t-butoxide ion,
Other bulky bases are triethylamine,
Bulk is important in determining the axial/equatorial positions of groups in cyclohexane rings.
The t-butyl group will always be equatorial.
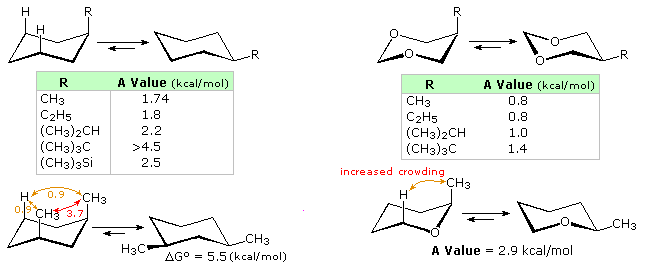
When two groups are competing for position, the bulkier group will be equatorial.


