Question #70429
1 Answer
Both ethyl chloride and allyl chloride react with aqueous sodium hydroxide at normal temperatures.
Ethyl chloride is a gas, and alkyl halides are relatively nonpolar.
You would have to do the reaction in a mixed solvent like aqueous acetone (acetone to dissolve the halide; water to dissolve the sodium hydroxide).
Ethyl chloride is a 1° halide, so it will react slowly by an
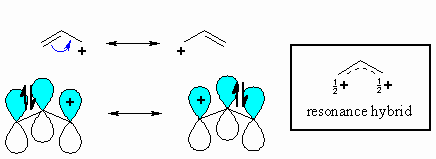
Allyl chloride is an allylic halide. It reacts much faster than ethyl chloride (by an