Question #cbe87
1 Answer
Here's how the nuclear equations would look for type of decay.
- Gamma emission
A gamma particle is just a photon, which in turn is a particle that has no mass and no charge. As a result, the nuleus that emits the gamma particle will remain unchanged.
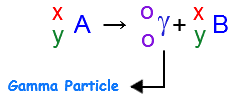
In your case, the nuclear equation will look like this
- Positron emission
Positron decay takes place when a proton inside the nucleus is converted into a neutron; at the same time, a positron and a neutrino are emitted.
A positron is the antiparticle of an electron, i.e. same mass, but a positive charge. Since a proton is converted into a neutron, the atomic number will decrease by 1, but the atomic mass will remain unchanged.
Notice that Br-80 decays into Se-80.
Electron capture takes place when an electron falls into the nucleus. As a result, a proton is converted into a neutron and a neutrino is emitted.
Once again, the conversion of a proton into a neutron will decrease the atomic number by 1, but keep the atomic mass unchanged.
Once again, Br-80 will decay into Se-80.

