Question #38e4f
2 Answers
Borazine has 12 σ bonds and 3 π bonds.
Borazine is an inorganic compound with the chemical formula B₃N₃H₆.
Three BH units and three NH units alternate in a cyclic structure similar to that of benzene.

Every single bond is a σ bond.
Every double bond consists of a σ bond and a π bond.
We count
- 3 B-N single bonds (3σ)
- 3 B-H single bonds (3σ)
- 3 N-H single bonds (3σ)
- 3 B=N double bonds (3σ + 3π)
TOTAL = 12 σ bonds + 3 π bonds
Borazine, or
To determine the number of sigma and pi bonds a molecule has, use its Lewis structure.
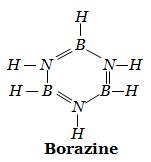
Keep in mind that each single bond is a sigma bond, and each double bond contains 1 sigma bond and 1 pi bond.
So, if you look at the above structure, you'll notice that it has 3 double bonds and 9 single bonds. This means that you'll get 3 pi bonds, one from each double bond, and 12 sigma bonds - 9 from each single bond and 3 from each double bond.
Therefore, borazine has 12 sigma and 3 pi bonds.


