Will you help me finish up this question?
2 .Many monatomic ions are found in seawater, including the ions formed from the following list of elements. Write the Lewis symbols for the monatomic ions formed from the following elements:
(a) K
(b) Mg
(c) Sr
(d) Br
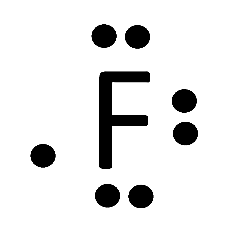
(e) F
2 .Many monatomic ions are found in seawater, including the ions formed from the following list of elements. Write the Lewis symbols for the monatomic ions formed from the following elements:
(a) K
(b) Mg
(c) Sr
(d) Br
(e) F
1 Answer
Here's what I get.
Explanation:
(a)
(b)
(c)
(d)
 )
)
(e)