What are the different kinds of f orbitals?
2 Answers
Explanation:
So there is only one kind of
So the
 )
)
With the different dimensions. Hope it helps.
They are quite complicated, and can often do combinations of
For an introduction into these kinds of bonds:
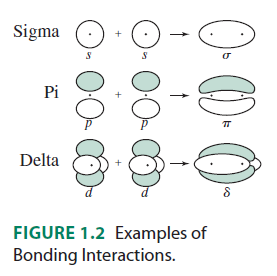
The
- The
#f_(z^3)# (#m_l = 0# ) is the only one that only#sigma# bonds. It can bond head-on along the#z# axis.
- The
#f_(y(3x^2 - y^2))# (#m_l = -3# ) can#sigma# bond along the#x# axes (for example, with a#p_y# orbital) AND#pi# bond along the#y# axes (for example, with a#p_x# orbital, or a#d_(xy)# orbital).
It can alternatively form a
#phi# bond (a six-lobed side-on overlap) along the#xy# plane (with another#f_(y(3x^2 - y^2))# orbital in a bimetallic complex).
- The
#f_(x(x^2 - 3y^2))# (#m_l = +3# ) can#sigma# bond along the#y# axes (for example, with a#p_y# orbital) AND#pi# bond along the#x# axes (for example, with a#p_x# orbital, or a#d_(xy)# orbital).
It can alternatively form a
#phi# bond (a six-lobed side-on overlap) along the#xy# plane (with another#f_(x(x^2 - 3y^2))# orbital in a bimetallic complex).
- The
#f_(yz^2)# (#m_l = -1# ) can form decent#sigma# bonds along the#y# axes, AND/OR#pi# bonds along the#y# AND#z# axes.
It can alternatively form a
#phi# bond (a six-lobed side-on overlap) along the#yz# plane (with another#f_(yz^2)# orbital in a bimetallic complex).
- The
#f_(xz^2)# (#m_l = +1# ) can form decent#sigma# bonds along the#x# axes, AND/OR#pi# bonds along the#x# AND#z# axes.
It can alternatively form a
#phi# bond (a six-lobed side-on overlap) along the#xz# plane (with another#f_(xz^2)# orbital in a bimetallic complex).
- The
#f_(z(x^2 - y^2))# (#m_l = -2# ) is for#pi# bonding along ANY of the axes,#x,y# , or#z# . The lobes lie above and below each of the axes, but also along them.
It can alternatively form a
#delta# bond with another#f_(z(x^2 - y^2))# orbital in a bimetallic complex.
- The
#f_(xyz)# (#m_l = +2# ) is for#delta# bonding along ANY of the planes (#xz, yz, xy# ) (for example, with#d_(xy)# ,#d_(xz)# , or#d_(yz)# orbitals).
It can alternatively form a
#pi# bond with another#f_(xyz)# orbital in a bimetallic complex.