Question #2d908
2 Answers
 )
)
Explanation:
You may want to draw the bonds between them. Just remember that the cation will be attracted to the anion.
It is difficult to draw the lattice structures of ionic compounds without a computer.
Explanation:
It is more important that you be able to recognize the unit cells in the lattice structures.
The positive and negative ions are often in a cubic array. Here are two examples.
The

The
The unit cell extends through the whole crystal.
This animation shows the

The green
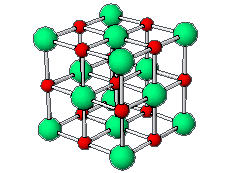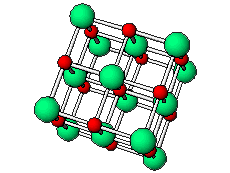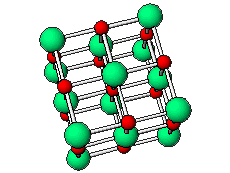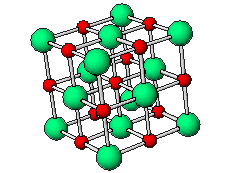
In the picture below, the

These unit cells extend through the whole crystal.




