Why is hydrogen atomic spectrum a line spectrum?
1 Answer
Jul 3, 2017
Continuous spectra are the entire visible electromagnetic radiation spectrum whereas line spectra are the characteristic wavelengths of radiation emitted as photons of each element.
Explanation:
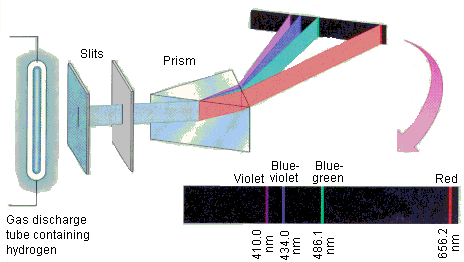
Each line on the spectrum is where

