Why does NH3 have a lower boiling point than H2O?
How would you explain this in terms of H-bonding?
How would you explain this in terms of H-bonding?
1 Answer
Well the simplest answer is...
Explanation:
As you know, the normal boiling points of water,
Hydrogen-bonding occurs when hydrogen is bound to a STRONGLY electronegative element, i.e.
Now for water, the hydrogen bonding network is particularly extensive...
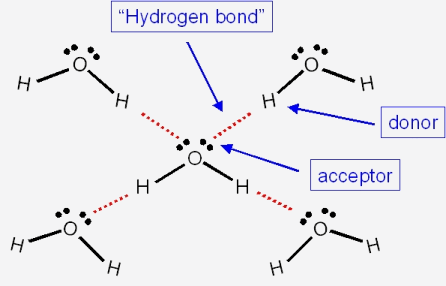
Whereas for ammonia....
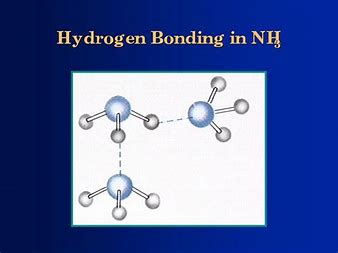
...given that the nitrogen ATOM has ONLY the one lone pair..the opportunity for intermolecular interaction via hydrogen-bonding is diminished with respect to water. This is reinforced by the polarity of an
And the end result? Well, we try to account for the experimental results....but certainly the normal boiling point is an excellent parameter with which to assess the intermolecular force.

