Why does acetone evaporate faster than ethanol yet still has a higher surface tension?
1 Answer
Nov 3, 2016
Acetone evaporate faster than ethanol because of Hydrogen Bond concept.
Explanation:
Acetone being a ketone has no direct
Therefore, more stroger physical bonds have to be destroyed in ethanol, than in acetone. Hence, acetone evaporates faster than ethanol inspite of having higher surface tension.
![Acetone] (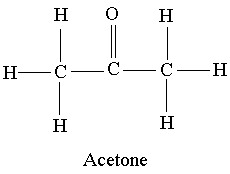 )
)
Ethanol
NOTE : To know more, please check into :
https://en.wikipedia.org/wiki/Hydrogen_bond.

