Where does reduction occur in a galvanic cell?
1 Answer
In a typical galvanic cell such as The Daniel Cell reduction occurs at the +ve electrode (anode).
Consider the standard electrode potentials:
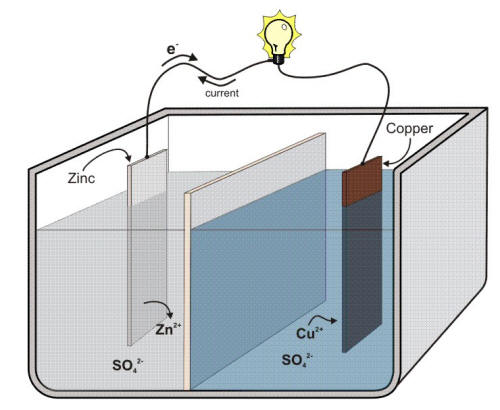
You can see that the potential of the zinc half cell is more negative than the potential of the copper half cell so electrons will flow from the zinc to the copper electrode.
This "potential difference" is measured in volts. We work it out by subtracting the least positive
So the maximum voltage we can get from the cell (emf) is:
0.34 - (-0.76) = 1.1v
So the overall cell reaction is:
Reduction is gain of electrons so this must be occuring at the copper electrode which is the anode:

