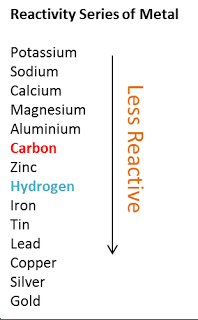
What is the outcome of reaction, of copper in HCl?
How would you describe the corrosion occurring?
How would you describe the corrosion occurring?
1 Answer
Oct 22, 2017
There will be no reaction.
Explanation:
There will be no reaction. Copper is a very unreactive metal, and it does not react with hydrochloric acid. It is above copper in a metal reactivity series, so copper cannot replace the hydrogen in