What is missing in the way electrons are shown in three-dimensional models of both the Bohr and Schrodinger atoms?
1 Answer
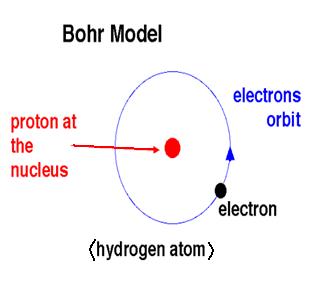
Bohr describes electrons as a particle that orbits a nucleus at a 'fixed' distance, thus resulting in circular patterns of orbit.
The Schrodinger model treats electrons as waves and not as particles that can be described as being in a particular location at a given time. The way Schrodinger describes electrons around an atom are shapes where electron density is predicted to be highest around an atom - these are known as 'cloud densities'. The actual 'location' of an electron is not shown, just an area where density is most probably. (This is derived from probability density from the Schrodinger equation).

So really, electrons are not treated as waves in Bohr's model but equally are not treated as particles in Schrodinger's model (which, outside of the quantum world of science, they can be assumed to be).
In truth, the Schroginer models biggest downfall is the fact that it is just that - a model. True understanding of quantum science doesn't lend itself to visual models at all, it is all far more abstract than any drawing can describe. We use the models, however, to allow a basic understanding to occur and to 'break-in' a students or persons understanding....
It is VERY important to be aware of the fact that all the above are just models - nothing is proven to be correct, just more or less likely. It is also worth noting that the Bohr model isn't exactly "wrong", it just isn't detailed enough for quantum studies and over-simplifies the idea of electron orbitals. But sometimes a broad understanding is all that is required and for the vast majority of chemical study / application, the Bohr model describes atomic trends in the periodic table and enables bonding models to be predicted.

