What is an example of oxidation and reduction?
1 Answer
Nov 20, 2015
Explanation:
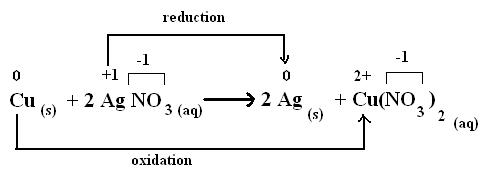
The following single replacement reaction is also a redox reaction.
The copper is oxidized so that its oxidation state increases from 0 to +2, and the oxidation state of silver is reduced from +1 to 0.