What element is represented by the electron configuration #1s^2 2s^2 2p^2#?
1 Answer
Jun 24, 2017
carbon (C)
Explanation:
The electron configuration tells us that there are 6 electrons in the element.
The superscripts indicate how many electrons are present in the orbital. For instance,
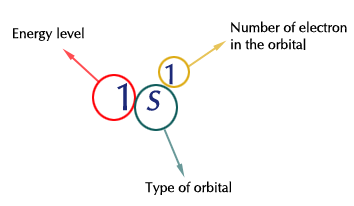
Since atoms are electrically neutral, the number of protons equals the number of electrons. So if you have an element with 6 electrons, it must have 6 protons.
The element with an atomic number of 6 is carbon.

