What are two characteristics of a molecule that make it suitable for analysis by infrared spectroscopy?
I know that one of them is that it must have a dipole but is there another?
I know that one of them is that it must have a dipole but is there another?
1 Answer
You are right that it has to have a dipole (not necessarily a net dipole). I'm not sure what other specific requirements you may be wondering about, but I can list several, and maybe one of them is what you are looking for.
REQUIREMENTS FOR VISIBILITY IN THE IR REGION
The main properties of a molecule that allow it to be analyzable via IR spectroscopy are:
#1)# It can experience a change in dipole moment, whether it is induced or permanent. We then must have that it is heteronuclear, if it is diatomic (therefore,#"N"_2# ,#"F"_2# , etc. are invisible in the IR).
#2)# It should have resonant frequencies that are in the infrared frequency range of#100 - 4000# #"cm"^(-1)# .
#3)# Ideally, it should be not overly soluble in a nonpolar solvent, which is ideal since many analyses are carried out using a solvent such as#"CS"_2# or#"CCl"_4# . If something is too soluble, one may saturate or overload the spectrometer.
CHANGE IN DIPOLE MOMENT
Requirement
A somewhat tricky example is
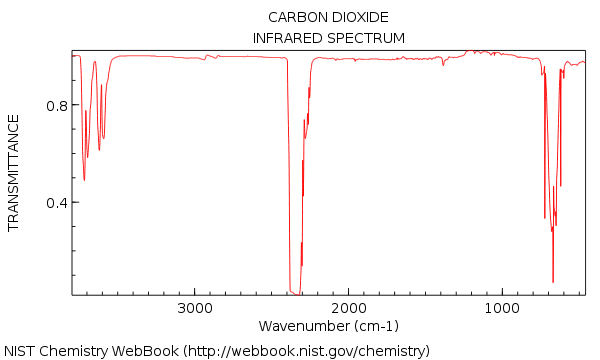
which you can estimate to be near
SOLUBILITY IN A NONPOLAR SOLVENT
Nice IR solvents that are commonly used are
This kind of consideration is not necessary, but being too soluble may make it frustrating to get a spectrum without overloading the spectrometer. Being "somewhat" soluble in nonpolar solvents is probably fine.

