What are the formula, molar mass, and color of potassium permanganate?
1 Answer
See explanation.
Explanation:
Potassium permanganate is the potassium salt of the permanganate anion,
Since potassium form
- one potassium cation,
#1 xx "K"^(+)# - one permanganate anion,
#1 xx "MnO"_4^(-)# 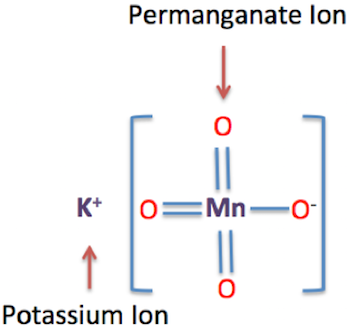
Therefore, the chemical formula for potassium permanganate will be
The molar mass of potassium permanaganate can be found by adding the molar masses of its constituent elements.
You will thus have
#M_ ("M KMnO"_ 4) = M_ ("M K") + M_ ("M Mn") + 4 xx M_("M O")#
Use the periodic table to write
#M_("M KMnO"_4) = "39.0983 g mol"^(-1) + "54.93085 g mol"^(-1) + 4 xx "15.9994 g mol"^(-1)#
#M_ ("M KMnO"_4) = "158.027 g mol"^(-1)#
Finally, potassium permanganate has a dark gray - bronze - purplish color in the solid state and a magenta color in solution.
 )
)

