What are the causes and effects of ozone depletion?
3 Answers
- there are many harmful effects of ozone layer depletion .
Explanation:
- harmful effects of ozone layer depletion are:
- damage to DNA and leads to mutation
- damage to skin cells
- ageing of skin
- cause various types of cancers
- snow blindness
- cataract
thus , ozone layer depletion cause very harmful effects.
See below.
Explanation:
Ozone depletion is primarily caused by human activities. The main effect of ozone depletion is an increase in UV-B rays reaching the earth's surface.
Causes : chlorofluorocarbon (CFCs), halons, and other compounds deplete the ozone layer. These chemicals are found in cleaning agents, aerosols, insulating foam, and refrigerants. CFCs and halons break down into chlorine and bromine which in turn destroy the ozone layer.
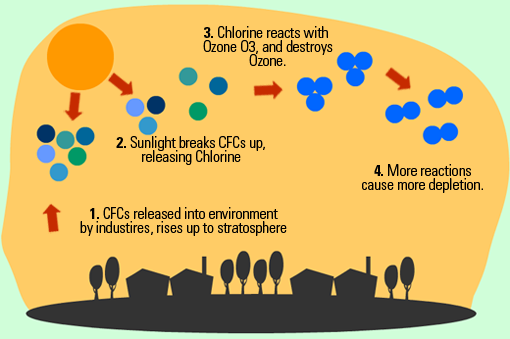
Effects :
Humans: an increase in UV-B rays means a higher risk of skin cancer, eye cataracts, and blindness. Read more here.
Marine life: Phytoplankton and zooplankton are very sensitive to the amount of light in their environment, and increases in UV-B rays would greatly affect them. Because these organisms are the base of the food chain, declines in their numbers would likely have wide-reaching effects for all marine life. Read more here.
Plants: UV-B rays negatively affect plants, including crops humans rely on. An increase in UV-B rays can mean smaller leaf size, decreased plant growth, and lower quality crops for humans. Plants form the basis for most food chains, thus negative effects would likely cascade to those organisms relying on them. Plants are also very important in terms of respiration, photosynthesis, soil stability, and a decline in plant productivity/reduced plant growth would potentially affect soil erosion and productivity and the carbon cycle. Read more here.
Ozone depletion is the breaking down of the earth's ozone layer. Ozone naturally absorbs UV, so we are more vulnerable without it.
Explanation:
The most famous example is aerosol cans. Chemicals used as propellant in aerosol cans react with ozone and break it down. This causes ozone depletion. Ozone naturally absorbs UV from the sun, making the earth more resistant against solar flares and everyday UV radiation. Worst case scenario, if we have no ozone left and a solar flare strikes earth, it would be much more damaging than if we had the ozone there to soak up some of the UV.
Hope this helps! Here are some links to read more:
http://www.ucsusa.org/global_warming/science_and_impacts/science/ozone-hole-and-gw-faq.html
http://www.conserve-energy-future.com/ozone-layer-and-causes-of-ozone-depletion.php



