What are strong and weak acids?
1 Answer
Strong acids are those that ionize completely in water and weak acids are those that only partially ionize in water.
Explanation:
Strong acids ionize completely in water and weak acids only partially ionize in water.
Strong acids completely break apart into their ions when dissolved in water. For example, hydrochloric acid,
Strong acids are strong electrolytes and will conduct electricity.
The strong acids are
Weak acids only partially break apart and will exist as a mixture of the acid and its ions. For example, acetic acid,
The reversible arrow show that as some of the acid forms its ions, there are also ions recombining to form the acid molecule.
Weak acids are weak electrolytes.
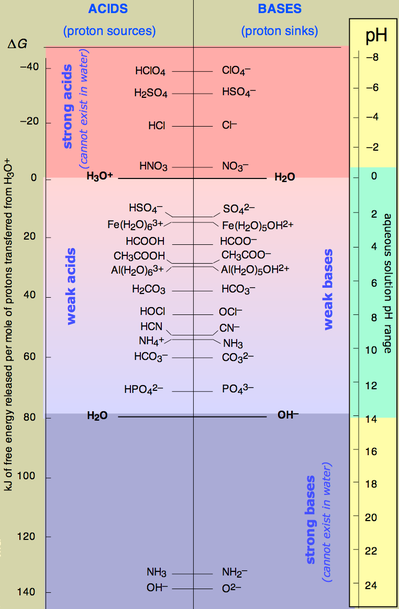
Most acids are weak by this definition. Don't make the mistake of thinking that they won't be corrosive because they are not strong.

