What are similarities of covalent and ionic bonding?
1 Answer
They both involve the bonded atoms receiving a full valence shell of eight electrons, called an octet, except for hydrogen atoms which receive a full valence shell with just two electrons, called a duet.
Explanation:
Covalent and ionic bonding both involve the formation of an octet of electrons in their valence shells, except for hydrogen which needs a duet of electrons. This results in the elements involved in the bonding to become stable.
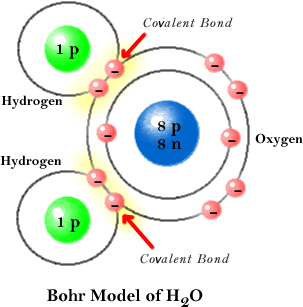
Covalent Bonding in a Water Molecule
The sharing of electrons between the two hydrogen atoms and the oxygen atom gives each atom a filled valence shell, two for each hydrogen atom and eight for the oxygen atom.
Ionic Bonding in a Sodium Chloride Formula Unit
The transfer of the outermost electron from the sodium atom to the chlorine atom, gives each eight electrons in their outer shells. This results in the sodium atom becoming a sodium cation, and the chlorine atom becoming a chloride anion. The oppositely charged ions form an ionic bond due to the electrostatic attraction between the ions.
 )
)

