Phosporous-32 is a radioisotope used in molecular biology After 44 days, 17% of the sample remains. How do you calculate the half-life?
1 Answer
Dec 5, 2016
You can do it like this:
Explanation:
The expression for 1st order decay is:
The graph looks like this:
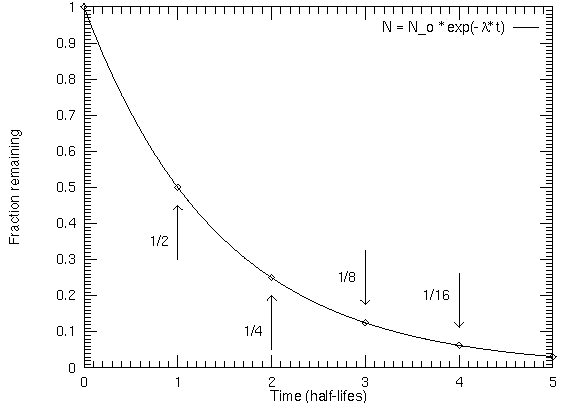
The key to the problem is to find the decay constant
Taking natural logs of both sides gives:
If we assume we start with 100 undecayed atoms this becomes:
This is related to the 1/2 life by:

