How many unpaired electrons are in a copper atom?
1 Answer
The electron configuration of copper is
An unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two spin states of the orbital specified by three quantum numbers n, l and m are fully occupied by two electrons).
To find out if the electrons inside an orbital are paired or not you need to find out the maximum electrons the subshell can hold. If the electrons in the subshell are less than the maximum electrons it can hold the electron in any of the orbital can be unpaired.
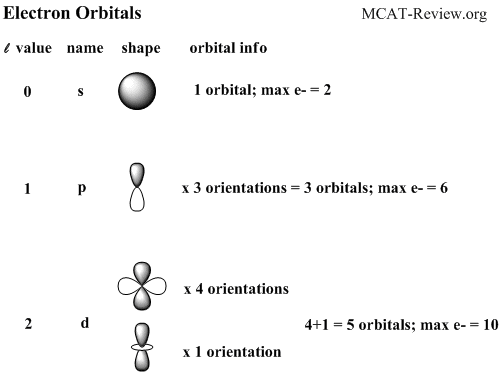
There is only 1 orbital in a s subshell . As an orbital can only hold 2 electrons a s subshell can hold a maximum of 2 electrons
Therefore the orbital in the 4s subshell is unpaired.
There are 3 orbitals in p subshell.
For p subshell the maximum electrons is 6. All the p subshells in this atom are full therefore no electron is unpaired.
There are 5 orbitals in d subshell
And for d subshell the maximum number of electrons is 10. There is only one d subshell in the copper atom and it is full.
Copper has only one unpaired electron


