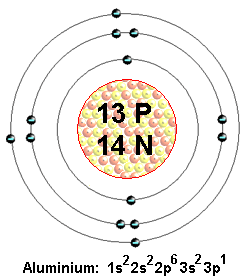
How many protons, neutrons, and electrons are present in an atom of aluminum-27?
1 Answer
Here's what I got.
Explanation:
Aluminium-27 is an isotope of aluminium characterized by the fact that is has a mass number equal to
Now, an atom's mass number tells you the total number of protons and of neutrons that atom has in its nucleus. Since you're dealing with an isotope of aluminium, it follows that this atom must have the exact same number of protons in its nucleus.
The number of protons an atom has in its nucleus is given by the atomic number. A quick looks in the periodic table will show that aluminium has an atomic number equal to
This means that any atom that is an isotope of aluminium will have
Since you're dealing with a neutral atom, the number of electrons that surround the nucleus must be equal to the number of protons found in the nucleus.
Therefore, the aluminium-27 isotope will have
Finally, use the known mass number to determine how many neutrons you have
#color(blue)("mass number" = "no. of protons" + "no. of neutrons")#
#"no. of neutrons" = 27 - 13 = 14#