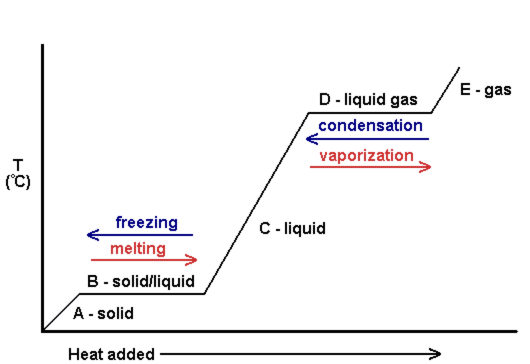
How is the temperature of a substance affected as it undergoes a change of state?
1 Answer
Jun 9, 2017
The temperature remains constant until the phase change is complete.
Explanation:
In the phase diagram below, you can see that B and D are phase changes, and the temperature is constant until the phase change is complete. The slanted lines represent the addition or removal of heat in between the different phases.