How do boiling points change at high altitudes?
1 Answer
At higher altitudes, boiling points are lower than at sea level becasue of the lower atmospheric pressure.
A substance boils when its vapor pressure equals the pressure of its surroundings. At sea level, atmospheric pressure equals 1 atm, which means that when a substance gets hot enough that its vapor pressure equals 1 atm, it starts to boil.
At higher altitudes, atmospheric pressure drops, as you can see here
 )
)
This means that the substance will not be as hot when boiling starts, since a lower atmospheric pressure implies a lower vapor pressure for boling, which in turn can be reached faster.
For example, let's take a look at water. At sea level, water boils at
By comparison, atmospheric pressure at sea level is 1 atm; at the top of Mount Everest, atmospheric pressure is 0.36 atm.
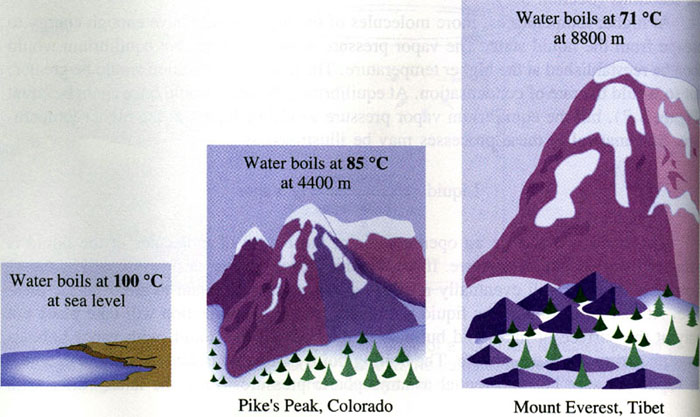
Here is a video demonstrating the fact that water will boil at lower temperatures when the atmospheric pressure is reduced.
video from: Noel Pauller


