The first step in drawing the Lewis dot structure for ethane (#C_2H_6#) is to determine how many valence electrons are available for the molecule.
Since #C# has 4 valence electrons, and each #H# atoms contributes 1 valence electron, the total number of electrons will be
#2*4 + 6*1 = 14# #"e"^(-)#
This means that the Lewis dot structure for #C_2H_6# must account for 14 valence electrons, either through bonding between atoms, or through lone pairs.
 )
)
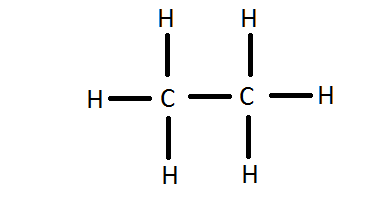
So, the two #C# atoms are placed in the center of the molecule. Each #C# atom forms three covalent bonds with three #H# atoms, with one aditional covalent bond being formed between the two #C# atoms.
Each of these seven single bonds contains 2 electrons, which means that a total of
#7 * 2 = 14# #"e"^(-)# were used for the #C_2H_6# molecule.
 )
) 
