How are acids and bases defined by Brønsted theory?
2 Answers
Apr 17, 2015
Easy question and easy answer. Look at the picture and you will see what are B-L acids and bases.

Apr 17, 2015
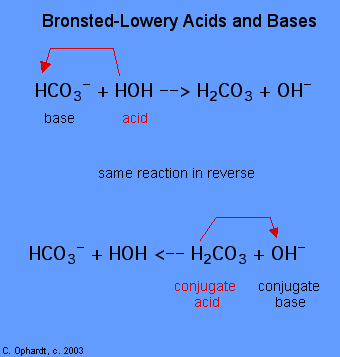

The fundamental concept of this theory is that when an acid and a base react with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchange of a proton (the hydrogen cation, or H+). This theory is a generalization of the Arrhenius theory.

