#"Fe"+"O"_2+"H"_2"O"="Fe"_2"O"_3 or "Fe(OH)"_3# Explain. What is the right answer?
1 Answer
Explanation:
This question concerns the corrosion of iron or rusting, which is known to be caused by the presence of air and water.
It is an electrochemical process. Consider the two 1/2 cells:
Electrochemical cells are set up on the surface of the iron. The
The iron forms
Water may contain a certain amount of dissolved oxygen. Its concentration determines which will be the sites of oxidation and reduction.
If you check the graphic, you can see what happens when a drop of water is in contact with the iron:
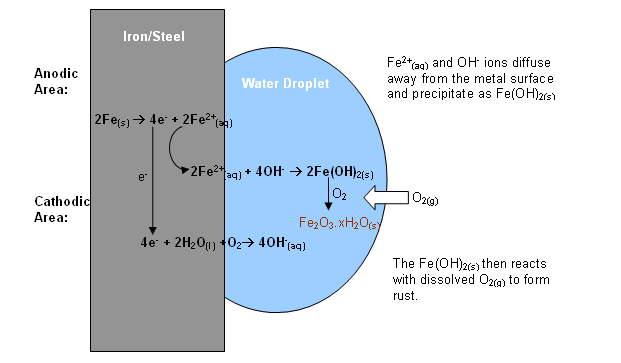
At the edges of the drop, where the concentration of dissolved oxygen is higher, oxygen is reduced to
The electrons come from the area of the iron which is near the centre of the drop where the concentration of dissolved oxygen is low. Iron is oxidised to
Overall the electrons are flowing from the centre of the metal surface to the edges of the drop.
This is responsible for "pits" of corrosion where the iron is exposed.
Unlike aluminium, where you have a protective oxide layer, the iron(II) and hydroxide ions migrate away from the surface of the iron and form iron(II) hydroxide:
This falls away from the surface of the iron and does not form a protective layer.
The iron(II) hydroxide then slowly oxidises to hydrated iron(III) oxide known as "rust".
In this experiment an agar plate has been impregnated with phenolphthalein and potassium ferricyanide. The former goes pink with
This shows an iron nail left for a few hours:
 )
)

