Can pi bonds rotate?
1 Answer
No, pi bonds cannot rotate.
When a pi bond is formed, all the pieces of a molecule are locked in place.
This is one reason cis- and trans- isomers are formed.
Cis -atoms locked on same side after pi bond formation.
Trans -atoms locked on opposite sides after pi bond formation.
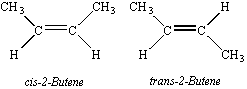
Notice how in Cisbutene,
The biggest change between, cis and trans isomers is the polarity of the molecule.
Cis molecules tend to be polar whereas trans molecules tend to be nonpolar.


