According to the activity series for metals, will the following reaction occur? #Cu(s) + HCl(aq) ->?#
1 Answer
Jun 15, 2017
The reaction will not take place. Hydrogen is above copper in the metal activity series, so hydrogen is more reactive than copper.
Explanation:
Activity Series for Metals
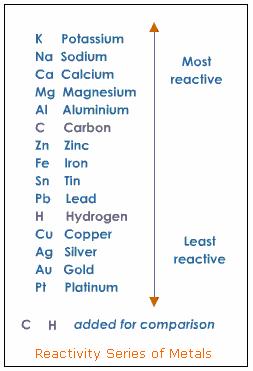
The video shows an experiment to determine the placement of three different metals (Cu, Zn and Mg) on the activity series.
Video from: Noel Pauller


