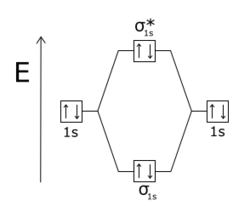
According to MO theory, what does the overlap of two s atomic orbitals produce?
2 Answers
Nov 19, 2016
It makes one
A simple depiction of these MOs is in an MO diagram:
And a depiction of
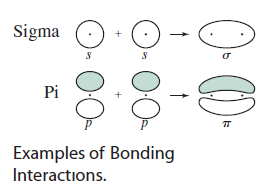
Nov 19, 2016
The overlap of 2 atomic orbitals produces molecular orbitals
Explanation:
The 2 MO are the bonding and the antibonding molecular orbitals