A tank of gas has partial pressures of nitrogen and oxygen equal to #1.61 xx 10^4# #"kPa"# and #4.34 xx 10^5# #"kPa"#, respectively. What is the total pressure of the tank?
1 Answer
Explanation:
The idea here is that when the volume and the temperature of a gaseous mixture are kept constant, the total pressure of the mixture is equal to the sum of the partial pressures of its gaseous components
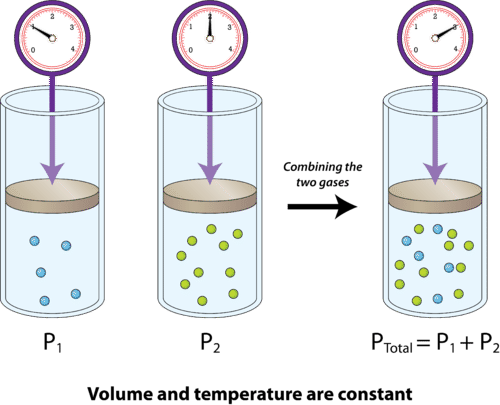
In your case, you know that the mixture contains nitrogen gas,
#P_"total" = P_ ("N" _ 2) + P_ ("O"_ 2)#
Plug in your values to find
#P_"total" = 1.61 * 10^4color(white)(.)"kPa" + 4.34 * 10^5color(white)(.)"kPa"#
#P_"total" = color(darkgreen)(ul(color(black)(4.50 * 10^5color(white)(.)"kPa")))#
The answer is rounded to two decimal places, the number of decimal places you have for your values.

