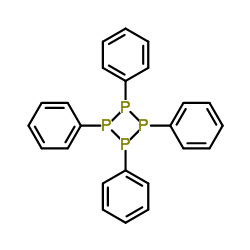
A solution of 2.50 g of a compound having the empirical formula #C_6H_5P# in 25.0 g of benzene is observed to freeze at 4.3°C . What is the molar mass of the solute and its molecular formula?
1 Answer
Here's what I got.
Explanation:
The idea here is that you need to use the freezing-point depression equation to determine what the molality of the solution.
Once you have the solution's molality, use it to find the number of moles of solute it contains.
As you know, the equation for freezing-point depression looks like this
#color(blue)(DeltaT_f = i * K_f * b)" "# , where
The cryoscopic constant of benzene is equal to
http://www.vaxasoftware.com/doc_eduen/qui/tcriosebu.pdf
You're dealing with a non-electrolyte, which means that the van't Hoff factor will be equal to
The freezing-point depression is defined as
#color(blue)(DeltaT_f = T_f^@ - T_f)" "# , where
Pure benzene freezes at
#DeltaT_f = 5.5^@"C" - 4.3^@"C" = 1.2^@"C"#
Plug in your values and solve for
#DeltaT_f = i * K_f * b implies b = (DeltaT_f)/(i * K_f)#
#b = (1.2 color(red)(cancel(color(black)(""^@"C"))))/(1 * 5.12 color(red)(cancel(color(black)(""^@"C"))) "kg mol"^(-1)) = "0.2344 mol kg"^(-1)#
As you know, molality is defined as moles of solute per kilograms of solvent.
#color(blue)(b = n_"solute"/m_"solvent")#
In your case, you have a mass of
#b = n_"solute"/m_"solvent" implies n_"solute" = b xx m_"solvent"#
#n_"solute" = "0.2344 mol" color(red)(cancel(color(black)("kg"^(-1)))) * 25.0 * 10^(-3)color(red)(cancel(color(black)("kg")))#
#n_"solute" = "0.00586 moles"#
Now, molar mass is defined as the mass of one mole of a substance. In your case, the
#M_M = "2.50 g"/"0.00586 moles" = "426.6 g/mol"#
As you know, a compound's empirical formula tells you what the smallest whole number ratio that exists between the elements that make up said compound is.
The molecular formula, which tells you exactly how many atoms of each element are needed to form the compound, will always be a multiple of the empirical formula.
In this case, calculate the molar mass of the empirical formula by adding the molar masses of all of its constituent elements
#6 xx "12.011 g/mol" + 5 xx "1.00794 g/mol" + 1 xx "30.974 g/mol" = "108.08 g/mol"#
You can thus say that
#108.08 color(red)(cancel(color(black)("g/mol"))) * color(blue)(n) = 426.6color(red)(cancel(color(black)("g/mol")))#
This will get you
#color(blue)(n) = 426.6/108.08 = 3.95 ~~ 4#
The compound's molecular formula will be
#("C"_6"H"_5"P")_color(blue)(4) implies "C"_24"H"_20"P"_4 -># 1, 2, 3, 4 - tetraphenyltetraphosphetane