Question #2975e
1 Answer
They have the same molecular formula, but belong to different homologous series.
Explanation:
Functional group isomerism is an example of structural isomerism – where a molecule has the same molecular formula, but a different arrangement of atoms in space.
In functional group isomerism, the atoms are arranged as such that the isomers belong to different families (homologous series ) of molecules.
Acetone and propionaldehyde are examples of functional group isomers, because they have the same molecular formula
Acetone is a ketone
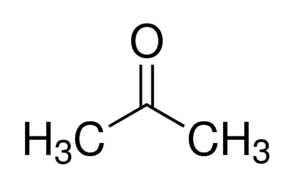
Propionaldehyde is an aldehyde
 )
)

