Question #fc45c
1 Answer
Explanation:
The idea here is that you need to use the molar mass of sucrose to calculate the number of moles present in your sample.
You know that sucrose has a molar mass of
You want to go from grams to moles, so set up the molar mass of the substance as a conversion factor that has moles in the numerator and grams in the denominator.
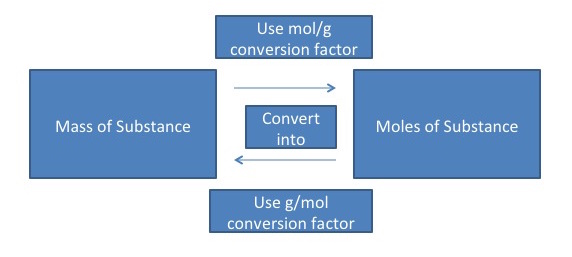
You should end up with
#45.4 color(red)(cancel(color(black)("g"))) * "1 mole sucrose"/(342.3 color(red)(cancel(color(black)("g")))) = color(darkgreen)(ul(color(black)("0.133 moles sucrose")))#
The answer is rounded to three sig figs.

