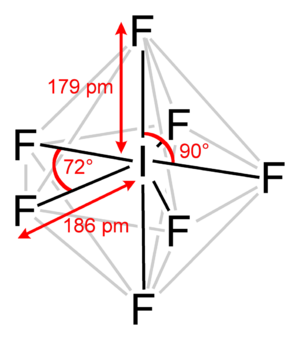
The interhalogen follows the normal application of #"VESPER"#, #"valence shell electron pair repulsion theory"#. The central atom, which is almost always the least electronegative atom......is iodine.
The most stable electronic arrangement to accommodate 7 bonding electron pairs is as a pentagonal bipyramid as shown.....
 )
)
Now such a geometry is conformationally mobile, and while I have never done the experiment, in the #""^(19)F{""^1H}# #"NMR spectrum"# at room temperature, we would probably see the one signal due to exchange between the axial and equatorial fluorines in a mechanism similar to Berry pseudorotation observed for #PF_5#.... And should the temperature be lowered to below a certain temperature, we would see the absorption decoalesce to give 2 signals in a 2:5 ratio (in a triplet and a sextet), the one representing the axial fluorine nuclei, and the other representing the fluorine atoms in the plane.
 )
) 
