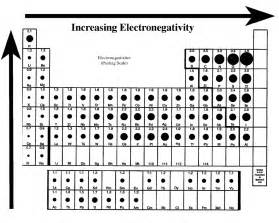
#"Electronegativity increases across a Period.........."#, from left to right as face the Table. And #"DECREASES"# down a Group, a column of the Periodic Table. See this [old answer.](https://socratic.org/questions/does-electronegativity-increase-or-decrease-when-you-go-a-period-on-the-periodic)
Given that you got #N,O,P,F#, clearly #F#, a #"Group 17 element"#, is the most electronegative element, followed by #"Group 16 oxygen"#, followed #"Group 15 nitrogen"#. And in last place of course is of course #"Group 15"# phosphorus, which comes from the next Period.
And so the order is #stackrel(F,O,N,P)"decreasing electronegativity"#.
 )
)
 )
) 
