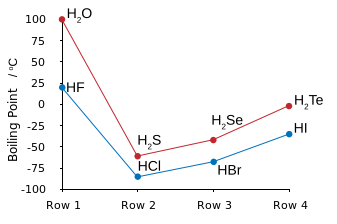
Which is the same thing as saying that #HCl#, and #HBr#, and #HI#, have LOWER boiling points than that of #HF#.
AS a chemist, as a physical scientist, it is your responsibility to assess data not remember them, and we list the NORMAL boiling points as follows:
 )
)
WHY are the boiling points of #HF# and #H_2O# so disproportionately high? The answer is intermolecular hydrogen bonding. That is the electronegative fluorine atom polarizes electron density towards itself to give a resultant molecular dipole, i.e. a separation of charge: #""^(-delta)F-H^(delta+)#.
In the condensed phase, these dipoles line up to give a strong contributor to intermolecular force: #""^(-delta)F-H^(delta+)*F-H*F-H# etc.
And thus #HF# is the LEAST VOLATILE hydrogen halide.
Note that hydrogen bonding also occurs for #HCl#, and #HI#, and also #H_2S#, but because the dipole is of smaller magnitude, hydrogen-bonding does not make such a contribution to intermolecular force. The boiling points here follow the order of dispersion force, which increase with the number of electrons, and thus with the atomic number of the halogen/chalcogen.
 )
) 
