What do we call the #O_2^(2-)# ion?
1 Answer
Aug 31, 2017
You gots
Explanation:
And a Lewis structure for
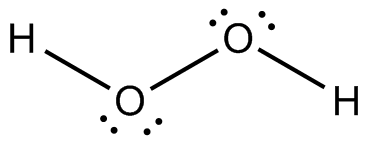
There were

