Question #021e1
1 Answer
Explanation:
One way we have of differentiating between various nuclides of the same element is by writing the name of the element followed by a hyphen and the mass number of the nuclide.
In your case, you would have zinc-66, a nuclide of zinc that has a mass number equal to
Now, an atom's mass number,
#color(blue)(ul(color(black)("mass number = no. of protons + no. of neutrons")))#
Alternatively, you can write
#A = Z + "no. of neutrons"#
Grab a Periodic Table and look for zinc,
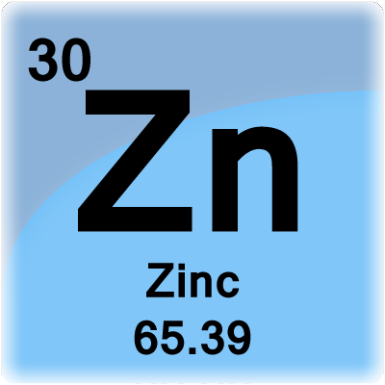
As you can see, zinc has
#Z = 30#
You already know from the nuclide's isotope notation that
#A = 66#
This means that you have
#color(darkgreen)(ul(color(black)("no. of neutrons" = 66 - 30 = 36)))#

