This is a good question, and it is of historical significance. These days laboratories tend to shy away from the use of mercury manometers to gauge pressure. Why? Well, there is the safety concern (we want to limit exposure to mercury!); but even despite this, there is the risk that if you break the container containing the mercury, you have a major clean up job. Spilled liquid mercury will inhabit every crack, and every cranny on a laboratory floor and bench.
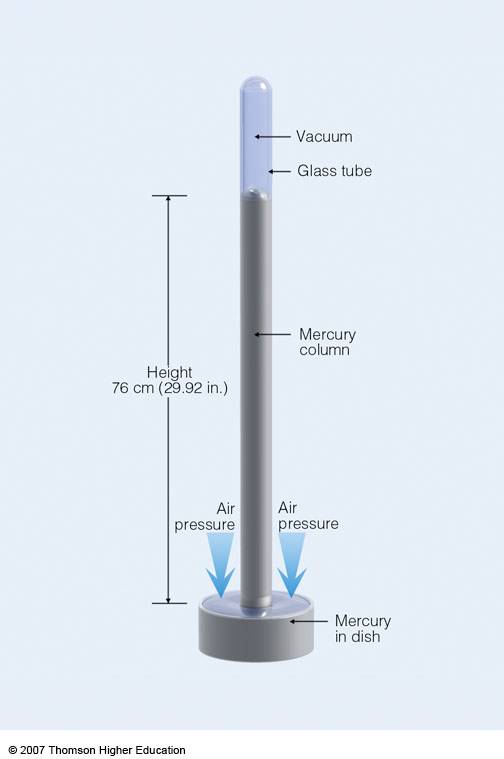
It is a FACT that #1*atm# of pressure, i.e. #101.3*kPa#, (i.e. a force per unit area) will support a column of mercury #760*mm*Hg# high. Often you see will manometers attached to vacuum lines, and when the vacuum is turned on, the column of mercury will snake up the thin glass column, the capillary, to give you a long, continuous column of mercury. This is a good physical representation of your vacuum. And if you isolate the line from DYNAMIC vacuum, you can see whether there is a leak in your line (i.e. put the line under static vacuum), because the level of mercury will drop down the column should there be a leak (usually the line has to be VERY WELL maintained if the level is not to leak overnight!).
With high vacuum devices, you used to be able to get mercury filled gauges to attach to your line, so-called McLeod gauges
 )
)
And these gauges measured pressures in #"millitor"# or less, i.e. #10^-3*mm*Hg#. Most labs that use high-vacuum pumps have tended to move away from mercury gauges. Mercury diffusion pumps, a very high vacuum device, that was easy to put together by a glassblower, have also given way to turbo-molecular pumps. Solid state electronic devices that measure pressure have also been introduced (God alone knows how these work, maybe some others will be able to explain!)
So finally, to your question. #1*"atmosphere"# #=# #101.3*kPa# #=# #760*mm*Hg#. And of course I found this older answer just now, not before I started this spray.
 )
)
 )
)  )
) 
