Question #97ddb
1 Answer
Apr 3, 2017
The
Explanation:
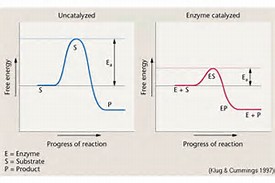
As shown on the diagram, a CATALYST can have an effect on the activation energy of a particular reaction. A catalyst has NO EFFECT whatsoever on
In the uncatalyzed reaction, the activation energy may be supplied by increasing the temperature, but it is essentially constant.

