How do I determine paramagnetic species in homonuclear diatomic molecules?
1 Answer
Apr 1, 2017
It is determined by looking at the molecular orbital diagrams. A blowby version is:
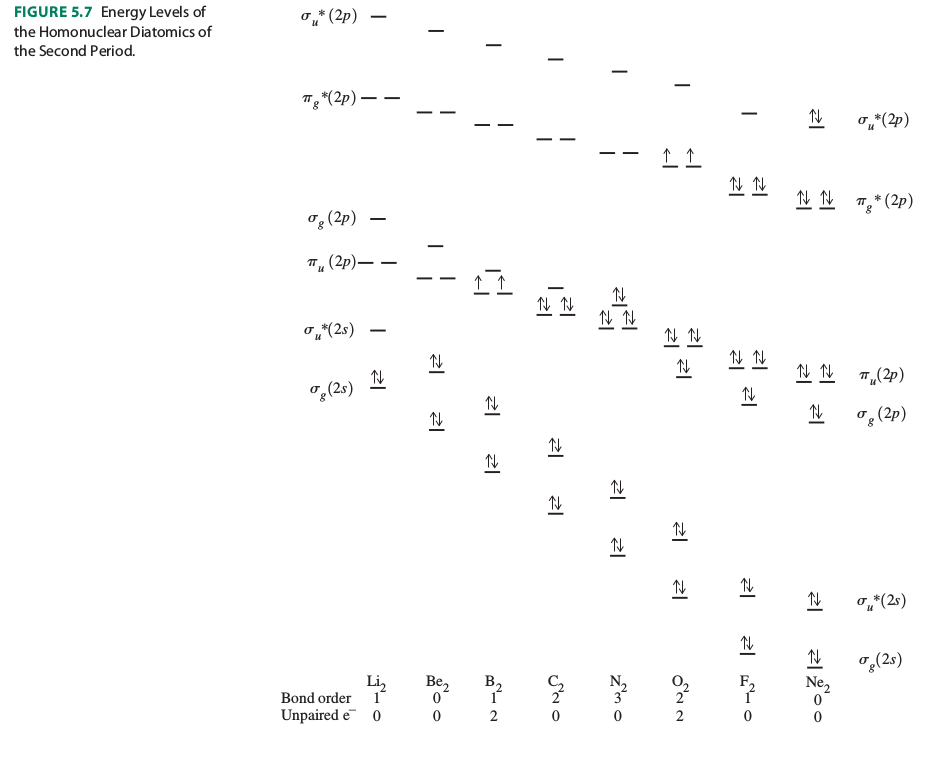
From these, which one has the unpaired electron(s)? That one is paramagnetic. Did you determine it to be

