Both molecules can support optical isomerism; but ONLY #"1,2-dimethylcyclohexane"# can support #"cis-trans isomerism"#.
For #"(b)"#, #"1,2-dimethylcyclohexane"#, CLEARLY, the pendant methyl groups can lie on the same side of the ring, or on opposite sides of the ring.
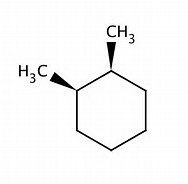
And this cis isomer, clearly has a plane of symmetry bisecting the #"MeCH-CHMe"# bond.
On the other hand, for #"trans-1,2-dimethylcyclohexane"#, this has a #C_2# axis that maps the pendant methyl group. And, thus, for a fact, this structure can support #"RR"# and #"SS"# enantiomers.
 )
)
For #"1,2-diethyl-1-methylcyclohexane"#, this generates 4 stereoisomers, because there are 2 chiral centres on the ring.
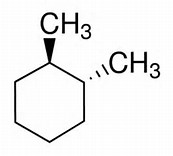 )
) 
