The saturated formula would be #C_8H_18#, and thus the given formula #C_8H_10# has #"4 degrees of unsaturation"#.
And each degree of unsaturation CORRESPONDS to an olefinic bond, i.e. #C=C# OR a RING JUNCTION.
 )
)
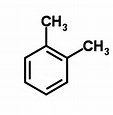
And thus the pictured #"xylene"#, as well as any one of its other 2 isomers (which are?) could correspond to the formula. And by my count #"xylene"# has #8xxC-C# #sigma# bonds, #10xxC-H# #sigma# bonds, and, formally, #3xxC-C# #pi# bonds.........
By joining and fusing rings, we could form a structure where the distinction between #sigma# and #pi# bonds are less ambiguous, however, these would be less common structures than the benzene derivative.
Note that the #"degree of unsaturation"# is a very useful criterion with which to assess organic molecules.
 )
) 
