How would successive ionization energies of a given element evolve?
1 Answer
Successive ionizations require the expenditure of greater energy input, each time an electron is removed from the valence shell.
Explanation:
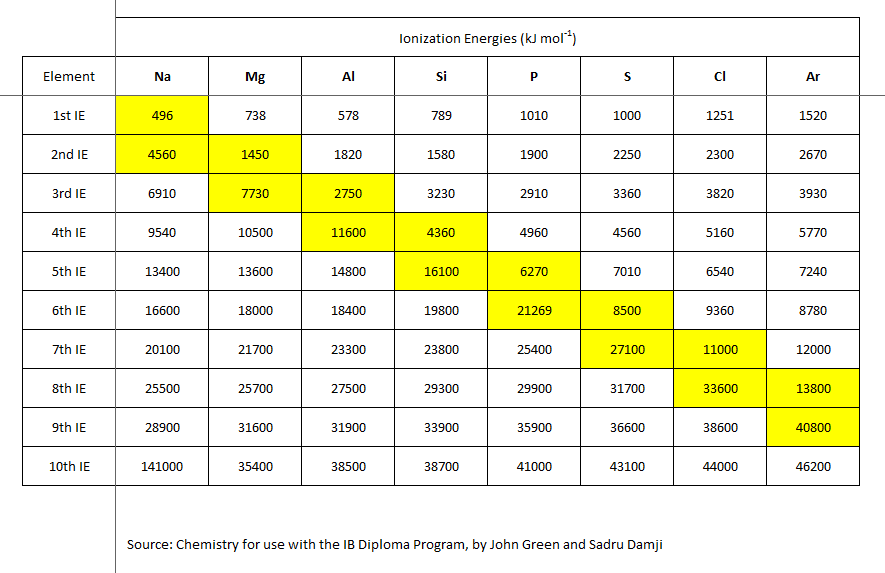
See here, #"........thomsonscience.files.wordpress.com/2011/09/screenshot-dataforionizationenergies-3rdperiodpng"#
A priori, we would expect that the ionization of a cation should require more energy than that required to generate the original cation:
The given data reflect this change. It also reflects the electronic structure of the atom. For magnesium metal the first 2 electrons are removed from the valence (outermost) shell; the third electron is removed from an inner non-valence core, and the VAST difference in ionization energy reflects this.

