When do nonbonding molecular orbitals form and what relative energy do they have compared to the original atomic orbitals?
1 Answer
This is basically a trick question; nonbonding orbitals are when orbitals cannot combine to form new molecular orbitals, and they just sit there. They CAN hold electrons, but they don't have to.
(With molecular orbital theory, you can account for empty molecular orbitals, but with valence bond theory, you cannot.)
The new "molecular orbitals" are really just the original atomic orbitals.
A simplified diagram of this kind of interaction would be, say, between a
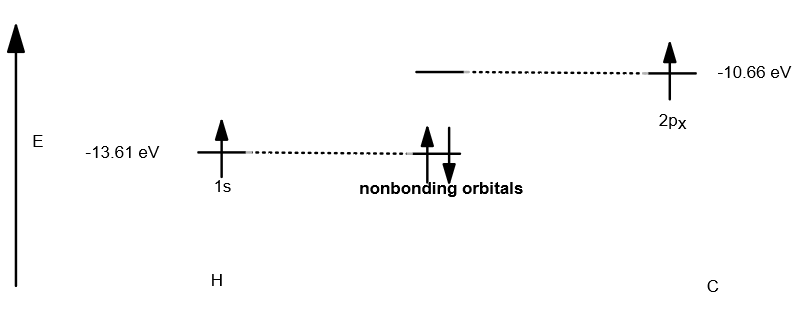
(this is a bond in isolation, which does not make physical sense. It is only for illustration of nonbonding interactions. In reality, carbon would hybridize its
Basically, nonbonding "molecular" orbitals are when two orbitals have the wrong "symmetry" (are not compatible), and thus, they cannot overlap successfully.
As a result, they remain as atomic orbitals and do not change from their original energy.

