Question #e2639
1 Answer
Sep 6, 2016
No, it is called dipole-dipole interaction, which is a weaker type of hydrogen bond.
Explanation:
Hydrogen bond occur only between hydrogen atoms and molecules possessing nitrogen, oxygen and fluorine.
Example,
H-bond between water molecules
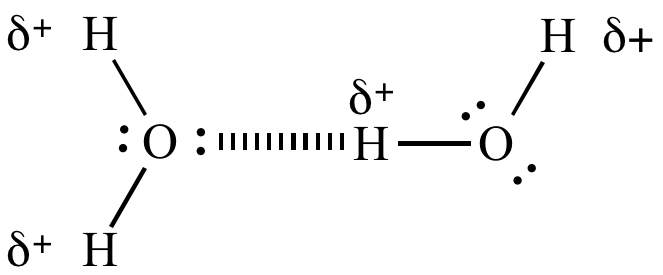
H-bond between ammonia molecules
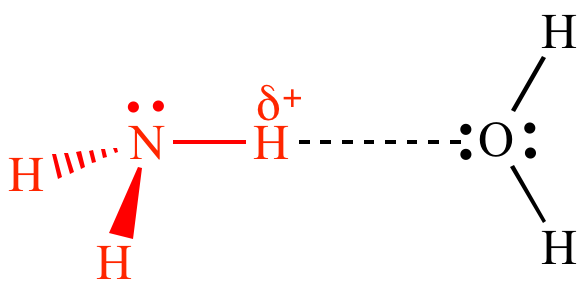
H-bond between hydrofluoric acid

The interaction between hydrochloric acid molecules
image source: http://johnsonapchem2015intervsintraforces.weebly.com

