Question #6ffde
1 Answer
Explanation:
Osmotic pressure is simply the pressure that must be applied to a solution in order to prevent the incoming flow of water through a semipermeable membrane.
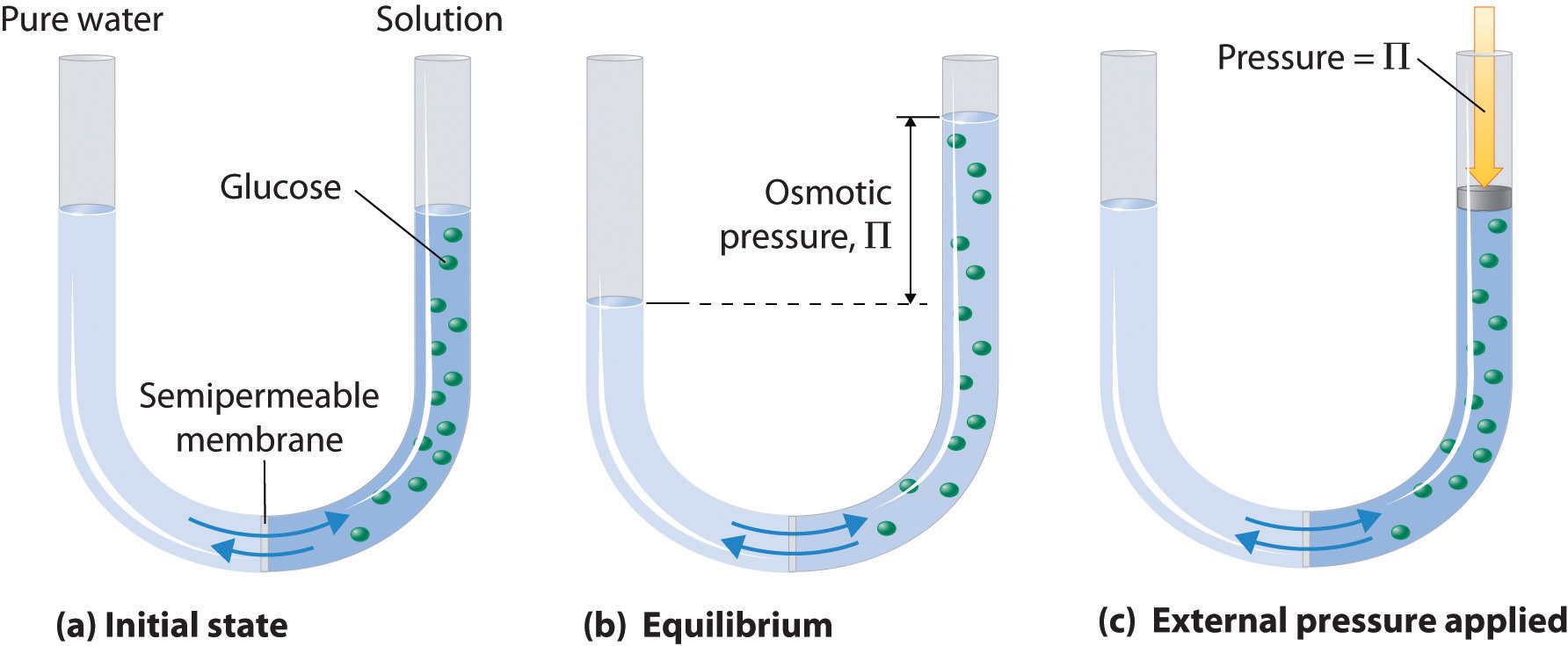
You can derive the equation that gives you the osmotic pressure of a solution that contains a non-electrolyte solute by using the ideal gas law equation
#color(blue)(|bar(ul(color(white)(a/a)PV = nRTcolor(white)(a/a)|)))" "# , where
Isolate
#P = n/V * RT#
Now, you know that the number of moles of solute per volume of solution gives you the molarity of the solution
#color(purple)(|bar(ul(color(white)(a/a)color(black)(c = n_"solute"/V_"solution")color(white)(a/a)|)))#
Plug this into the above equation to get the osmotic pressure,
#color(blue)(|bar(ul(color(white)(a/a)Pi = c * RTcolor(white)(a/a)|)))#
Now, let's say that
#n = (30.0 color(red)(cancel(color(black)("g"))))/(M_Mcolor(red)(cancel(color(black)("g")))"mol"^(-1)) = 30.0/M_Mcolor(white)(a)"moles"#
You thus have
#Pi = 30.0/M_M * 1/V * RT#
Isolate
#M_M = 30.0/Pi * (RT)/V#
#M_M = (30.0 color(red)(cancel(color(black)("moles"))))/(0.0167color(red)(cancel(color(black)("atm")))) * (0.0821(color(red)(cancel(color(black)("atm"))) * color(red)(cancel(color(black)("L"))))/(color(red)(cancel(color(black)("mol"))) * color(red)(cancel(color(black)("K")))) * (273.15 + 25)color(red)(cancel(color(black)("K"))))/(1.00 color(red)(cancel(color(black)("L"))))#
#M_M = "43,919"#
I'll leave the answer rounded to three sig figs and expressed in scientific notation
#M_M = color(green)(|bar(ul(color(white)(a/a)color(black)(4.39 * 10^4 "g mol"^(-1))color(white)(a/a)|)))#

