Question #c9ca2
1 Answer
Explanation:
From the data on the BCC structure I will work out the mass of 1 sodium atom.
Then I will work out the volume of the FCC unit cell, from which I will work out the density.
BCC refers to "body centred cubic". The unit cell is the smallest unit that, when repeatedly stacked together, will generate the entire structure.
A ball and stick model looks like this:

The lines joining the atoms are not bonds in the usual sense. They represent points of contact. Not all the atoms lie inside the unit cell.
There is 1 atom in the centre and at each vertex there is 1/8 of an atom.
This means that the unit cell contains 1 + (8 x 1/8) = 2 atoms.
The side of the unit cell is of length a which we can now calculate.
This will enable us to find the volume of the unit cell which will be
The direction from a corner of a cube to the farthest corner is called the body diagonal (bd).
The face diagonal (fd) is a line drawn from one vertex to the opposite corner of the same face.
Imagine looking at the unit cell in the plane of the screen from the direction shown:
You can see that a right - angled triangle is formed.
Using Pythagoras:
From the diagram we can say also that:
From
The atoms that lie along the body diagonal (bd) touch each other. From the geometry we can say:
Where R is the radius of the atom.
Putting in the numbers:
This is the length of the unit cell.
So the volume V of the unit cell is given by:
The mass m of the unit cell is given by:
We have already worked out that there are 2 atoms in the unit cell so the mass
The unit cell for face centred cubic (FCC) looks like this:
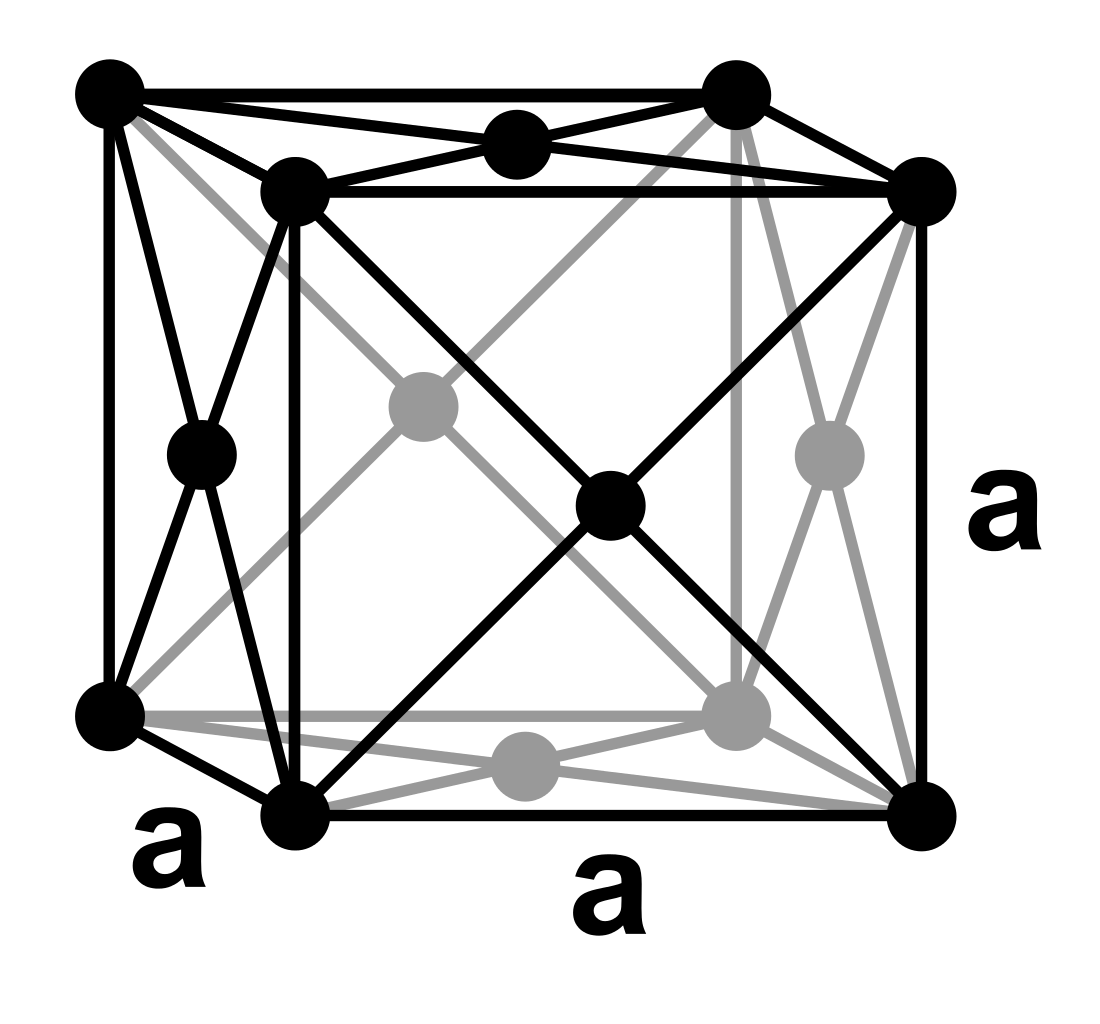
You can see that there is an atom at the centre of each face of the unit cell.
Each vertex contains 1/8 of an atom. Each face contains 1/2 of an atom.
So the total no. of atoms in the unit cell will be (8 x 1/8) + (6 x 1/2) = 1 + 3 = 4 atoms.
This can also be seen in the space filling model:

In the diagram the face diagonal is labelled b.
We can use Pythagoras to find a
Since the atoms are touching we can say that:
Where r is the atomic radius.
From Pythagoras:
We have shown that there are 4 atoms in the unit cell so we can write:
You can see that there is a slight increase in density at this very high pressure as FCC is a more closely packed arrangement.

